Pms Plan Template - Citemed’s proposed psur template comprises the following detailed sections.
Citemed’s proposed psur template comprises the following detailed sections.
Citemed’s proposed psur template comprises the following detailed sections.
(PDF) EU postmarket surveillance plans for medical devices
Citemed’s proposed psur template comprises the following detailed sections.
EU postmarket surveillance plans for medical devices Pane 2019
Citemed’s proposed psur template comprises the following detailed sections.
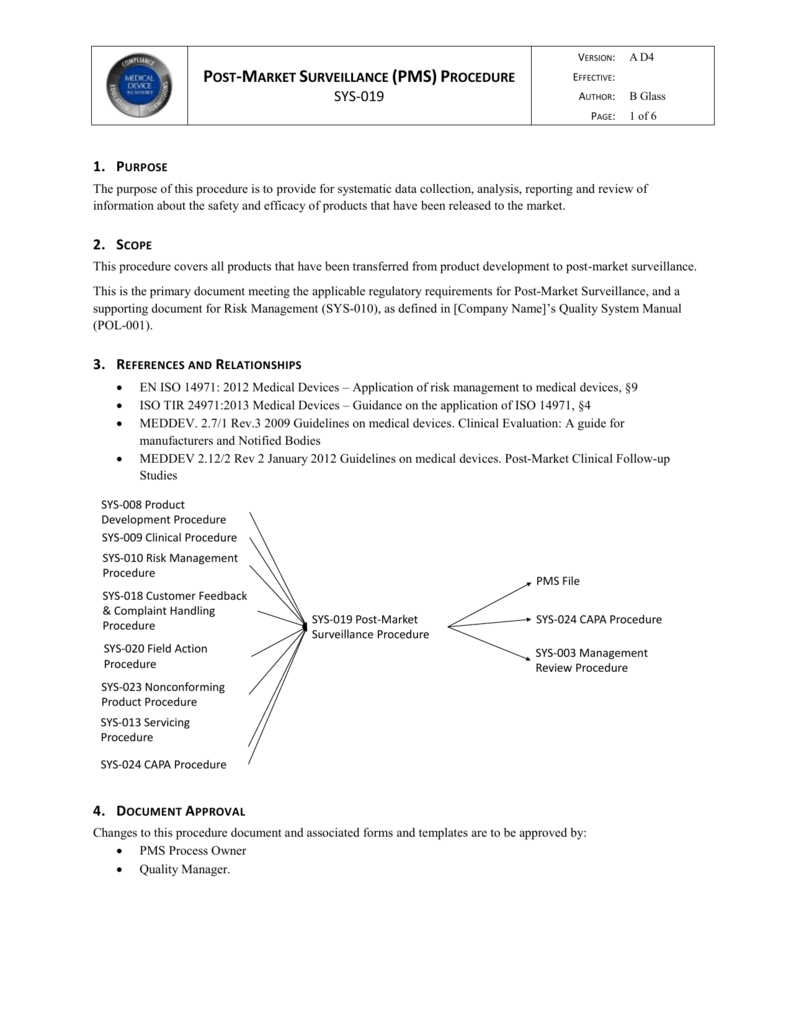
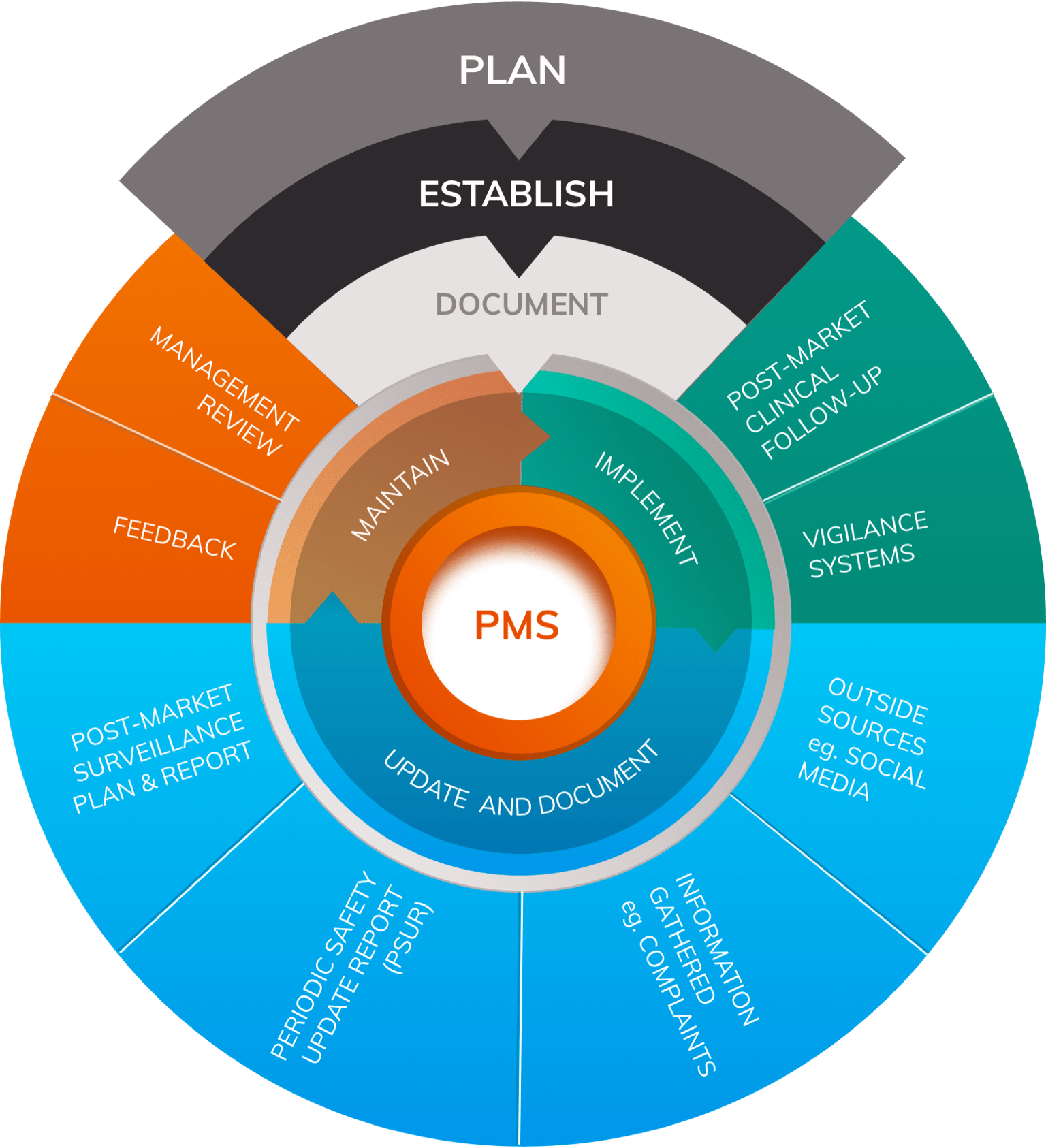
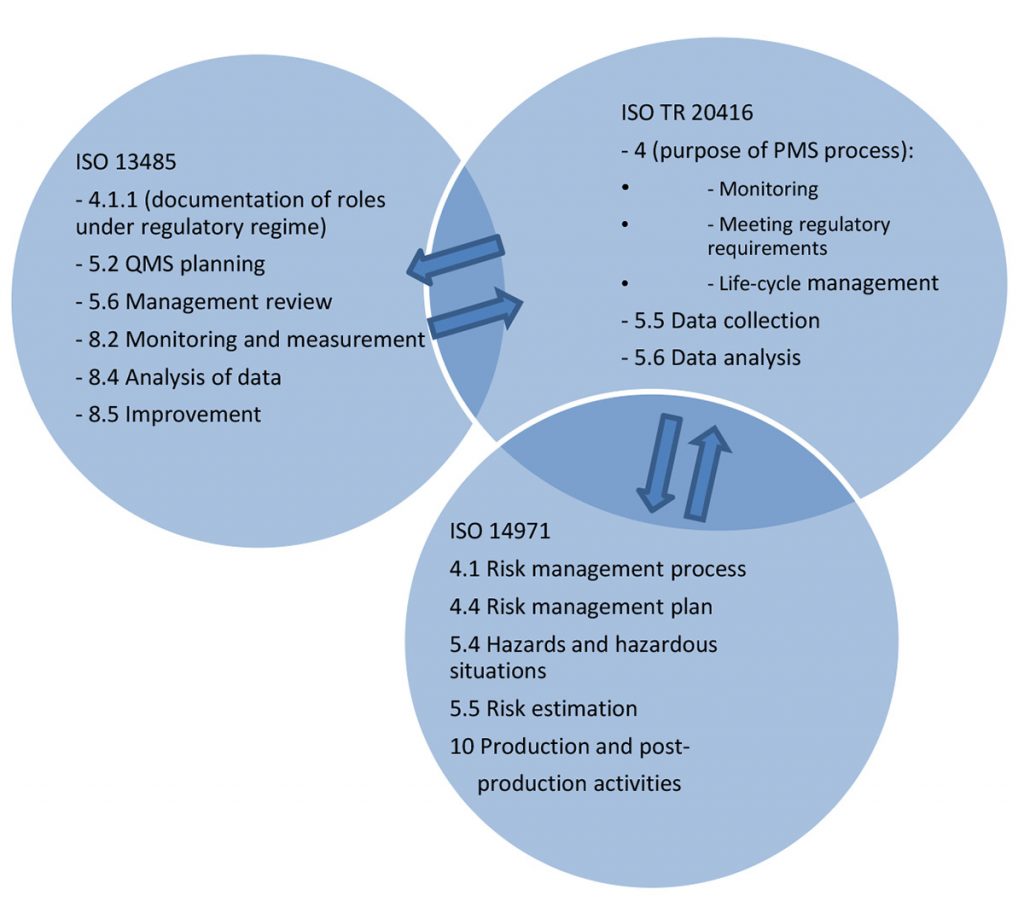
Postmarket surveillance is in itself a monitoring and measuring
Citemed’s proposed psur template comprises the following detailed sections.
PMS Plan Template R1 PDF Medical Device Business
Citemed’s proposed psur template comprises the following detailed sections.
Post Market Surveillance Plan PMS Plan Template
Citemed’s proposed psur template comprises the following detailed sections.
postmarket surveillance plan
Citemed’s proposed psur template comprises the following detailed sections.
PostMarket Surveillance (PMS) of medical devices
Citemed’s proposed psur template comprises the following detailed sections.
Practical advice for setting up an MDR and IVDR compliant PMS Plan
Citemed’s proposed psur template comprises the following detailed sections.
Post Market Surveillance Plan PMS Plan Template
Citemed’s proposed psur template comprises the following detailed sections.