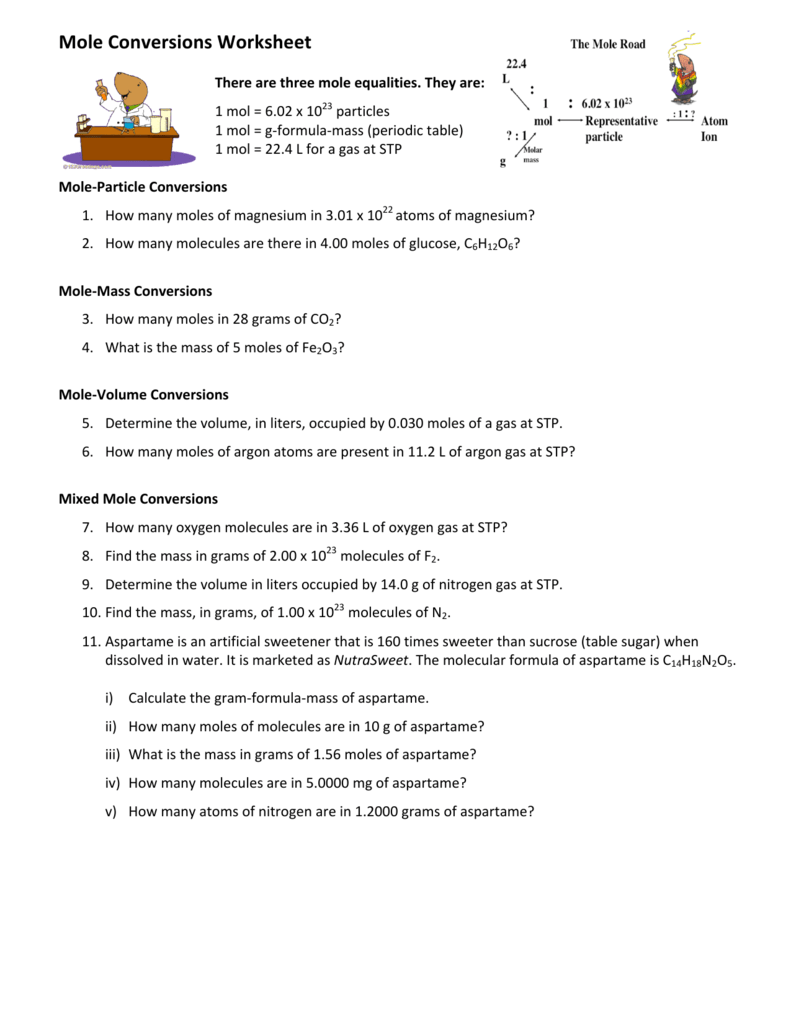
Moles To Particles Worksheet With Answers - While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. How many moles of magnesium is 3.01 x 1022 atoms of. Complete the following practice problems for mole conversion. How many moles are in 72.9 g of hcl? Elements generally exist as the particles we. Show your work and units! A) how many molecules are. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Each definition can be written as a set of two conversion factors.
Show your work and units! A) how many molecules are. Complete the following practice problems for mole conversion. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Each definition can be written as a set of two conversion factors. How many moles of magnesium is 3.01 x 1022 atoms of. Elements generally exist as the particles we. Mole worksheet #2 make the following conversions using unit analysis. How many moles are in 72.9 g of hcl? 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry.
There are three definitions (equalities) of mole. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. How many moles of magnesium is 3.01 x 1022 atoms of. Use a separate piece of paper, show all work, and circle your. Mole worksheet #2 make the following conversions using unit analysis. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Show your work and units! Elements generally exist as the particles we. How many moles are in 72.9 g of hcl? Complete the following practice problems for mole conversion.
Mole To Particle Conversion Worksheets
A) how many molecules are. How many moles of magnesium is 3.01 x 1022 atoms of. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. There are three definitions (equalities) of mole.
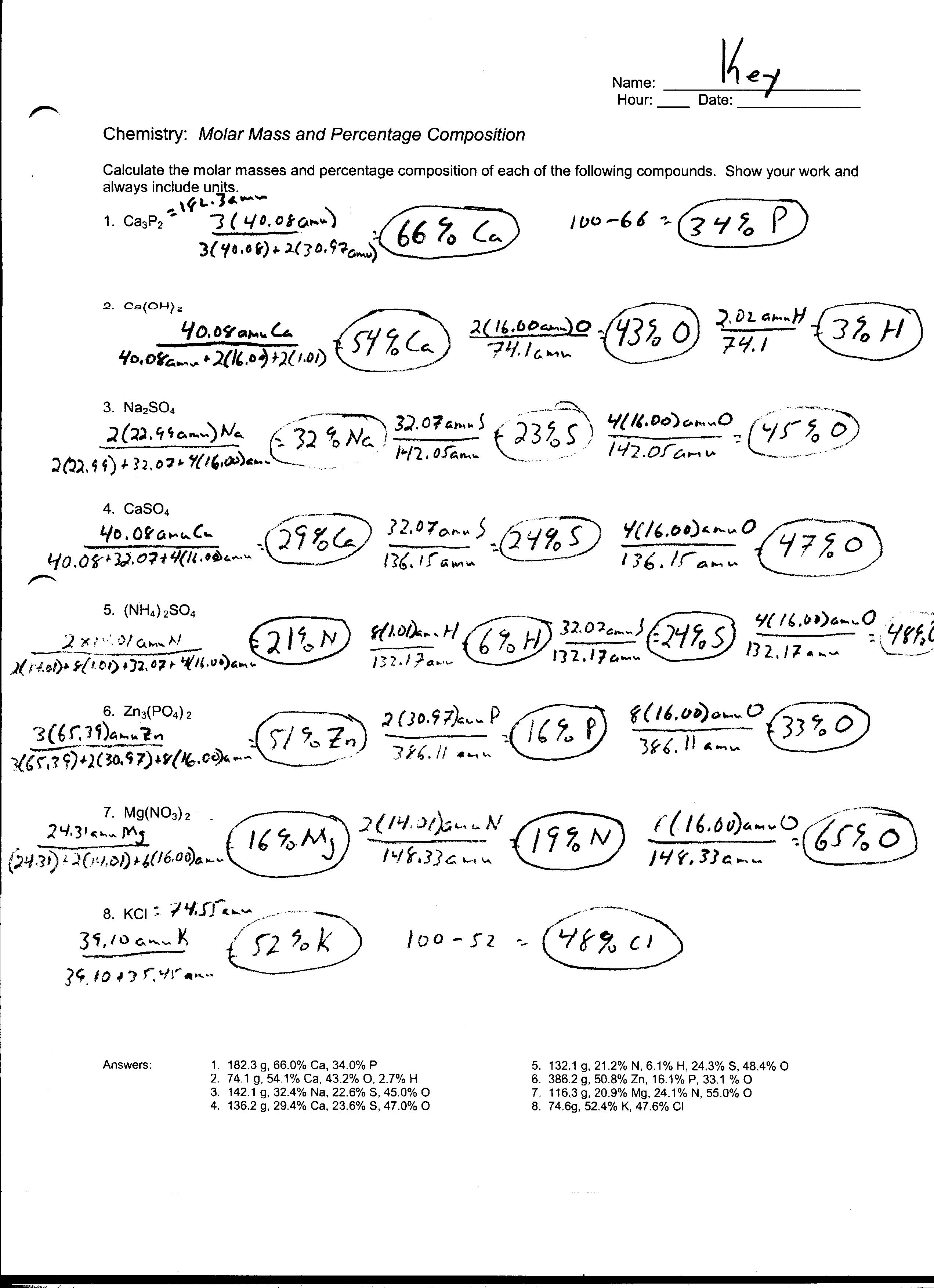
Moles And Particles Worksheet Mole To Gram Stoichiometry (mo
Show your work and units! Complete the following practice problems for mole conversion. How many moles of magnesium is 3.01 x 1022 atoms of. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Mole worksheet #2 make.
Grams Moles Calculations Worksheets
Each definition can be written as a set of two conversion factors. Complete the following practice problems for mole conversion. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. How many moles of magnesium is 3.01 x.
Mole Worksheet moles to particles Docsity
Use a separate piece of paper, show all work, and circle your. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. A) how many molecules are. How many moles are in 72.9 g of hcl? Worksheet #5.
Mole To Particle Conversion Worksheets
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. Each definition can be written as a set of two conversion factors. Complete the following.
Mole Conversions Worksheet Working With Moles And Particles
Use a separate piece of paper, show all work, and circle your. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. There are three definitions (equalities) of mole. How many moles of magnesium is 3.01 x 1022.
The Mole Avogadro's Number Worksheets
Each definition can be written as a set of two conversion factors. Show your work and units! A) how many molecules are. Mole worksheet #2 make the following conversions using unit analysis. Use a separate piece of paper, show all work, and circle your.
Mole Mass And Particle Conversion Worksheet
There are three definitions (equalities) of mole. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Complete the following practice problems for mole conversion. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do.
Mole Particle Conversions Worksheets
Worksheet #5 calculating number of particles, number of moles, and molar mass 1. How many moles of magnesium is 3.01 x 1022 atoms of. A) how many molecules are. There are three definitions (equalities) of mole. Mole worksheet #2 make the following conversions using unit analysis.
The Mole & Volume Worksheet Walkthrough Worksheets Library
Mole worksheet #2 make the following conversions using unit analysis. Each definition can be written as a set of two conversion factors. Show your work and units! While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Worksheet #5 calculating number of particles, number of moles, and molar mass 1.
A) How Many Molecules Are.
Elements generally exist as the particles we. Complete the following practice problems for mole conversion. Mole worksheet #2 make the following conversions using unit analysis. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry.
How Many Moles Of Magnesium Is 3.01 X 1022 Atoms Of.
Show your work and units! Worksheet #5 calculating number of particles, number of moles, and molar mass 1. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Each definition can be written as a set of two conversion factors.
How Many Moles Are In 72.9 G Of Hcl?
There are three definitions (equalities) of mole. Use a separate piece of paper, show all work, and circle your.