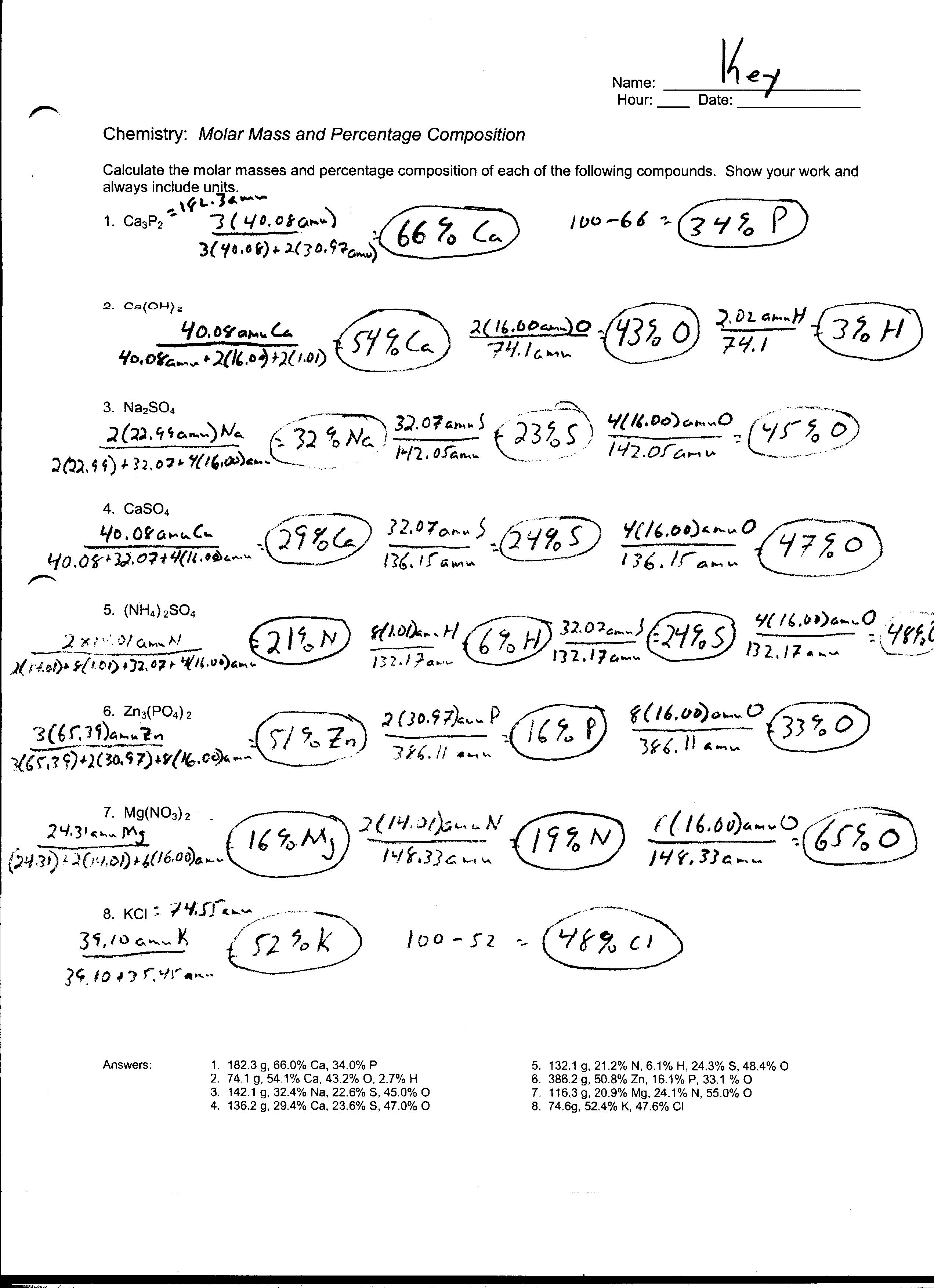
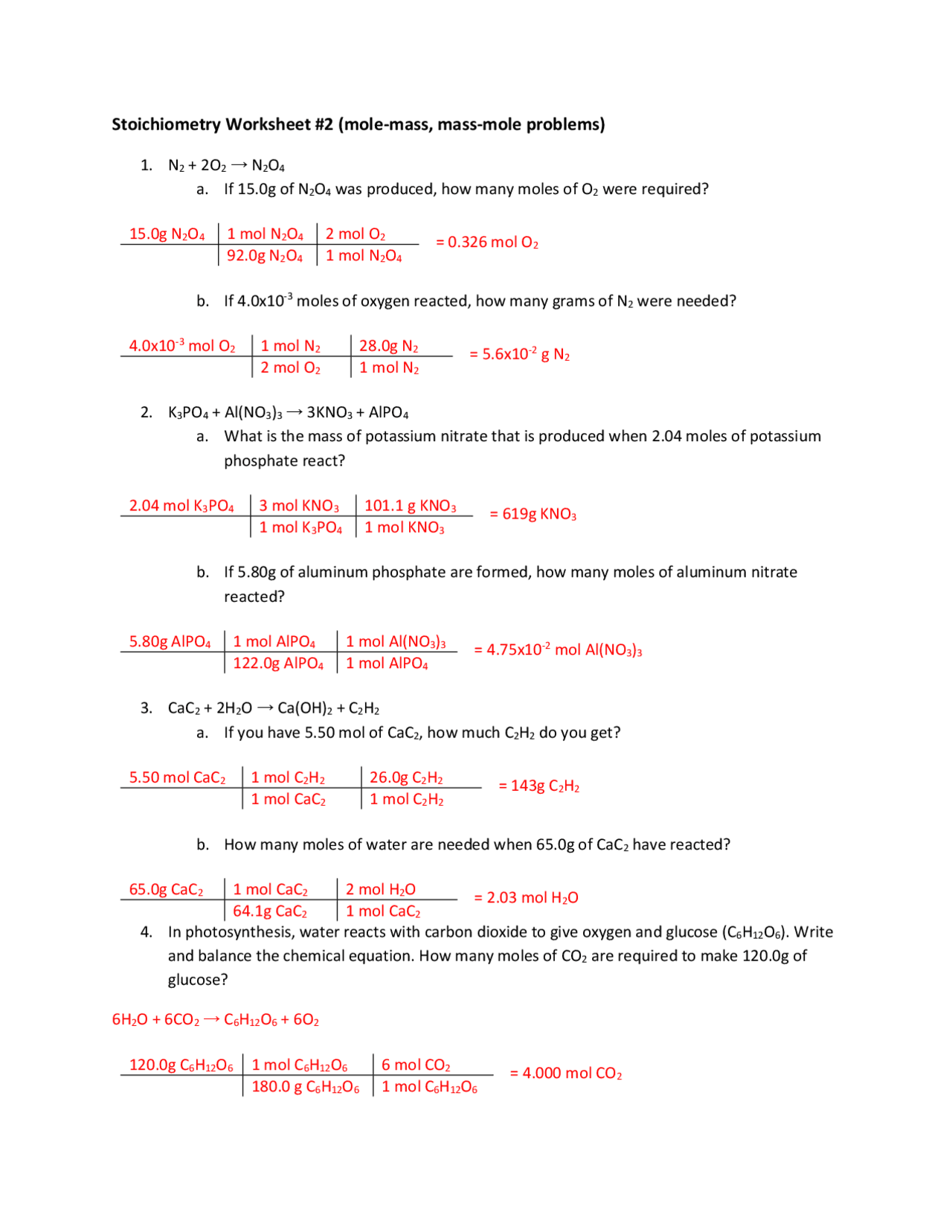
Mole To Mole Stoichiometry Worksheet - How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of.
How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1.
Unit 7 Stoichiometry Mole Conversion Worksheets
Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. How many moles of magnesium is 3.01 x 1022 atoms of.
Mole To Mole Stoichiometry Worksheets With Answers
How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1.
Mole Mole Stoichiometry Worksheets Answers
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of. Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Solved MoleMole Stoichiometry Worksheet Complete each of
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Mole Stoichiometry Worksheet
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Stoichiometry and The Mole Review Worksheet Set (Study Guide
Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Free Printable Mass to Mole Stoichiometry Worksheets Worksheets Library
Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. How many moles of magnesium is 3.01 x 1022 atoms of.
Mole Mole Stoichiometry Worksheets
Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Mole To Mole Stoichiometry Worksheets
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole Mole Stoichiometry Worksheets
Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Mole to mole problems 1.
Magnesium Reacts With Hydrochloric Acid According To The Following Balanced Chemical.
Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical.