Intermolecular Forces Practice Worksheet - Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to.
Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: What is the strongest intermolecular force present for each of the following molecules? You may find it useful to. 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force.
You may find it useful to. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide.
Chemistry Intermolecular Forces Practice by Teach Simple
What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find.
3. intermolecular forces worksheet with key
Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: What is the strongest intermolecular force present for each of the following molecules? 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. You may find.
Chapter 10 Practice worksheets What type(s) of intermolecular
1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: You may find it useful to. Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. What is the strongest intermolecular force present for each of.
Intermolecular Forces Practice Worksheets
Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. You may find it useful to. What is the strongest intermolecular force present for each of the following molecules? 1) hydrogen (h 2) london dispersion forces.
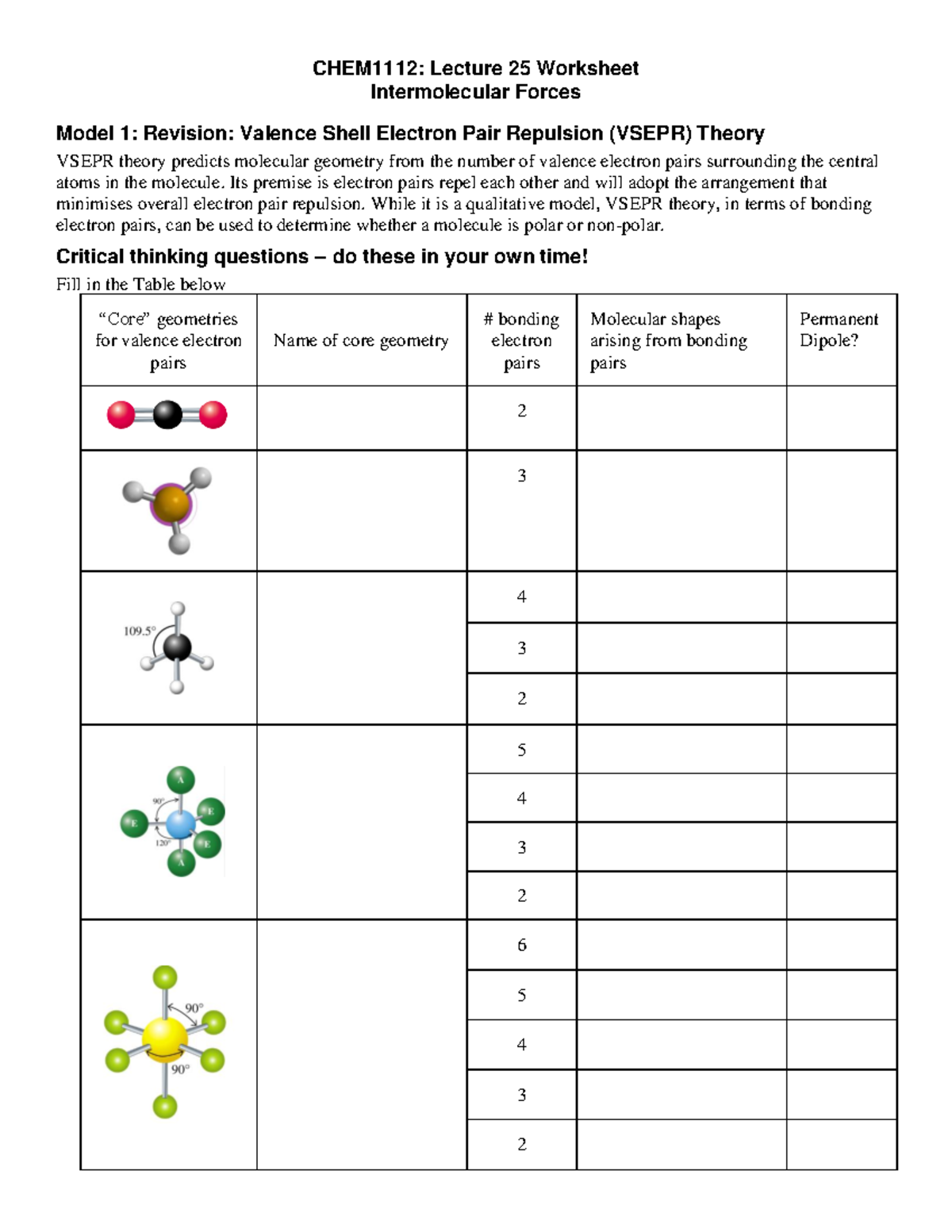
Worksheet 25 Lecture practice CHEM1112 Lecture 25 Worksheet
1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. What is the strongest intermolecular force present for each of the following molecules? Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: You may find.
Intermolecular Forces Worksheet Worksheet
Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. What is the strongest intermolecular force present for each of the following molecules? 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to. Tell which member of each of the following pairs of compounds you would expect to have the.
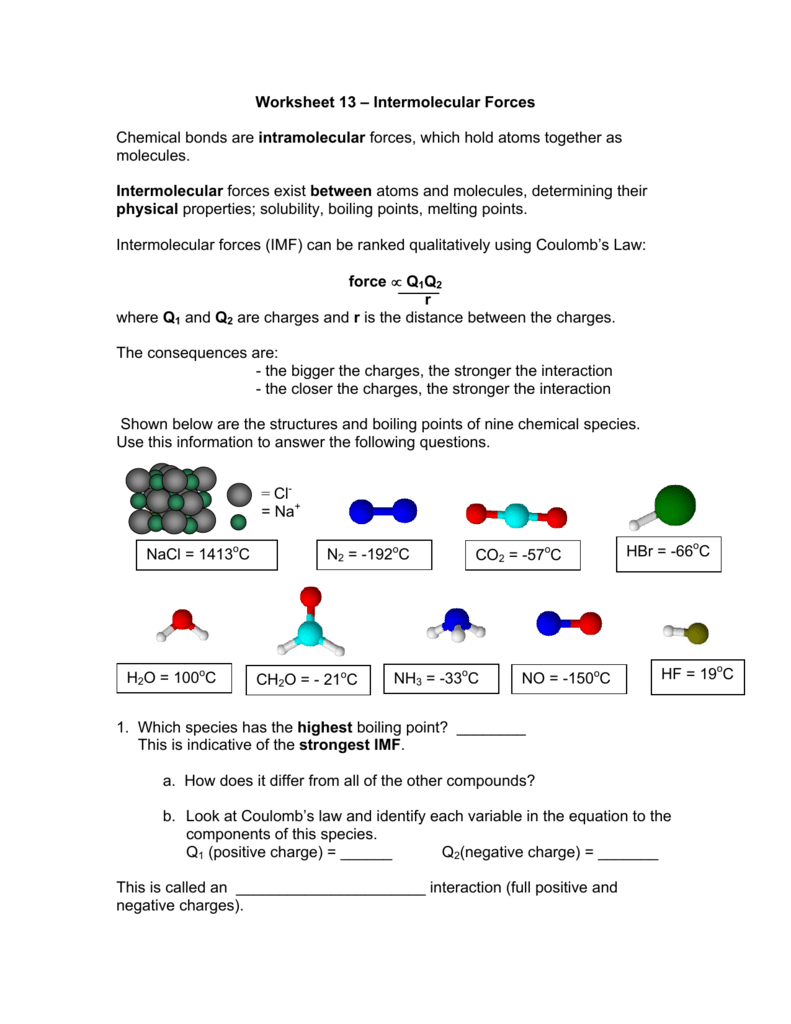
Worksheet 13 Intermolecular Forces Chemical bonds are
1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: You may find.
Intermolecular Forces Summary, Worksheet, And Key Worksheet
Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. You may find it useful to. What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the.
Intermolecular Forces Worksheet
What is the strongest intermolecular force present for each of the following molecules? Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: You may find.
Intermolecular Forces Worksheet And Answers Ch U4 A3 Intermo
Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: 1) hydrogen (h 2) london dispersion forces 2) carbon monoxide. What is the strongest intermolecular force present for each of the following molecules? You may find.
1) Hydrogen (H 2) London Dispersion Forces 2) Carbon Monoxide.
Intermolecular forces worksheet for each of the following compounds, determine the main intermolecular force. What is the strongest intermolecular force present for each of the following molecules? You may find it useful to. Tell which member of each of the following pairs of compounds you would expect to have the higher boiling point: