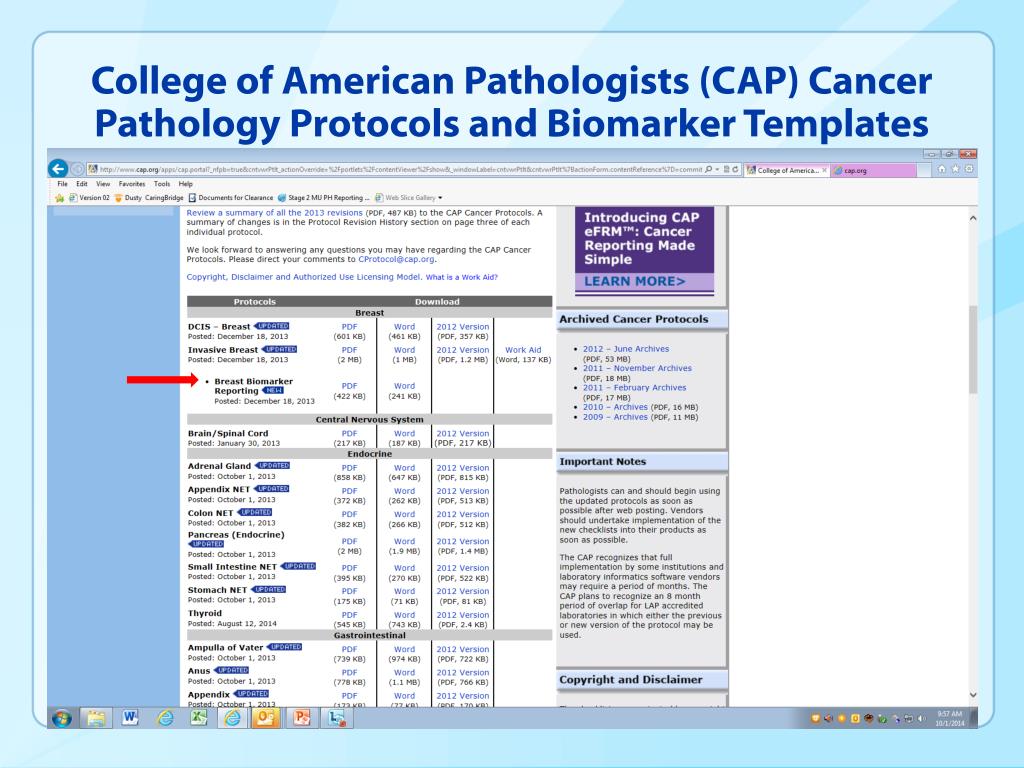
Cap Protocol Templates - This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker reporting protocols, as well as. The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports. Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. September 2022 select a single response unless otherwise indicated. For accreditation purposes, only the. Reporting template protocol posting date: June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with.
This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. Reporting template protocol posting date: For accreditation purposes, only the. The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports. Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker reporting protocols, as well as. June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with. September 2022 select a single response unless otherwise indicated.
This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. Reporting template protocol posting date: The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker reporting protocols, as well as. Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. September 2022 select a single response unless otherwise indicated. For accreditation purposes, only the. The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports. This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with.
Cap Cancer Templates
Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. September 2022 select a single response unless otherwise indicated. June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with. The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker.
Cap Cancer Template
June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with. This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Reporting template protocol posting date: For accreditation purposes, only the. Cap cancer protocols provide structure and data elements for consistent and.
(PDF) Standard 4.6 The Importance of CAP Protocols and DOKUMEN.TIPS
The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker reporting protocols, as well as. September 2022 select a single response unless otherwise indicated. This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. Reporting template protocol posting date: The electronic versions of over 100 cancer protocols.
Fillable Online CAP Cancer Protocol Adrenal Gland College of American
The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports. Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. For accreditation purposes, only the. This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. The college of.
Cap Cancer Templates
Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker reporting protocols, as well as. For accreditation purposes,.
Esophagus CAP protocol Esophagus Esophageal Cancer
September 2022 select a single response unless otherwise indicated. Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with. The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker.
PPT Cancer Pathology and Biomarker Reporting PowerPoint Presentation
Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. For accreditation purposes, only the. The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker reporting protocols, as well as. This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. The.
Cap Protocol Templates
September 2022 select a single response unless otherwise indicated. Reporting template protocol posting date: For accreditation purposes, only the. June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with. This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast.
CAP_Foundation_Cancer_Protocols_June2023.pdf
Reporting template protocol posting date: Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports. For accreditation purposes, only the. June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with. The college of american pathologists (cap) offers free printable versions of its cancer reporting and.
Fillable Online CAP Cancer Protocol Stomach Fax Email Print pdfFiller
The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports. June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with. This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. September 2022.
June 2021 This Biomarker Template Is Not Required For Accreditation Purposes But May Be Used To Facilitate Compliance With.
Reporting template protocol posting date: For accreditation purposes, only the. The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports. September 2022 select a single response unless otherwise indicated.
This Protocol Can Be Utilized For A Variety Of Procedures And Tumor Types For Clinical Care Purposes.
The college of american pathologists (cap) offers free printable versions of its cancer reporting and biomarker reporting protocols, as well as. This document provides a template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Cap cancer protocols provide structure and data elements for consistent and meaningful information in pathology reports.