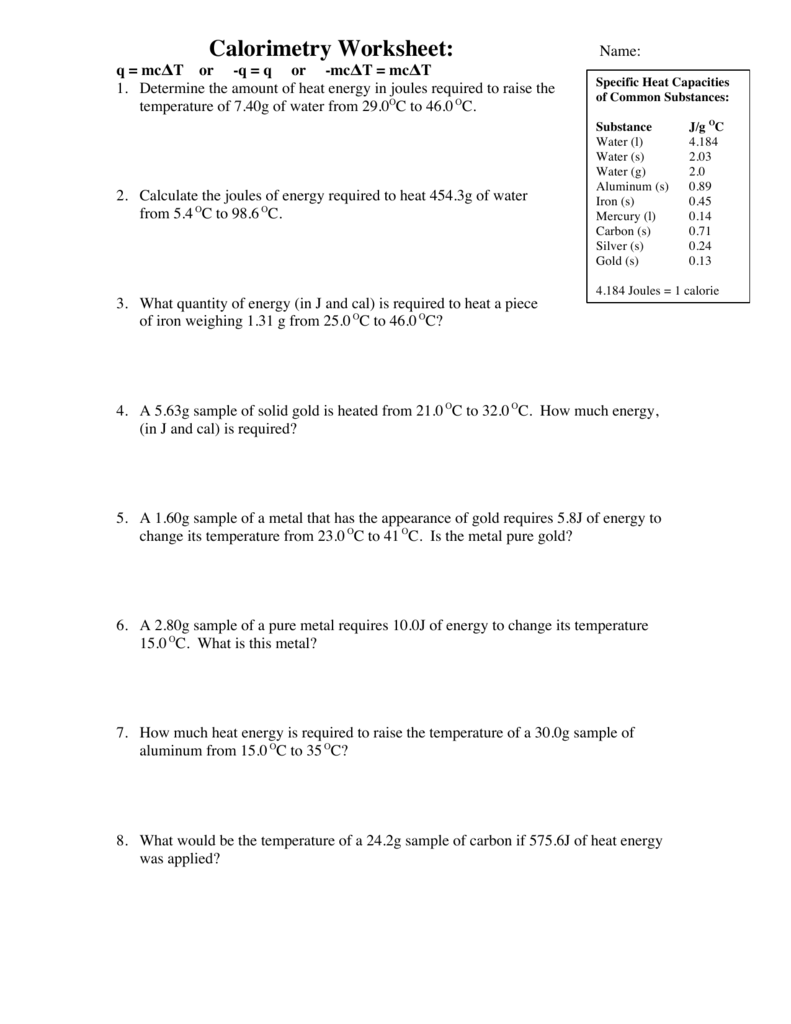
Calorimetry Problems Worksheet Answers - If the combustion of 0.285 moles of this compound causes the. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calorimetry questions and problems 1.
If the combustion of 0.285 moles of this compound causes the. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry questions and problems 1. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water.
If the combustion of 0.285 moles of this compound causes the. Calorimetry questions and problems 1. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water.
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library
Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calorimetry and molar enthalpy worksheet answer.
50 Calorimetry Worksheet Answer Key
1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If the combustion of.
Calorimetry Problems Worksheet With Answers
Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry questions and problems 1. 1) a compound is burned in a bomb calorimeter that.
Calorimetry Worksheet with Answer Key Exercises Chemistry Docsity
Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Calorimetry and molar enthalpy worksheet.
Calorimetry Worksheet Answers Pogil Printable Word Searches
If the combustion of 0.285 moles of this compound causes the. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in..
Calorimetry Questions And Answers
Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. 1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits,.
Chemistry Calorimetry Problems 1 Answers
The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. If the combustion of 0.285 moles of this compound causes the. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in.
Calorimetry Worksheet 1 Answers Heat Capacity Calorimetry Worksheet
Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a.
Calorimetry Practice Problems With Answers Calorimetry Pract
Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively. Problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned.
SOLUTION Phys1020 unit 5 worksheet 1 specific heat calorimetry Studypool
1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If the combustion of 0.285.
Calorimetry 1) If A Gold Ring With A Mass Of 5.5 G Changes Temperature From 25.0 C To 28.0 C, How Much Energy (In.
1) a compound is burned in a bomb calorimeter that contains 3.00 l of water. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the. The specific heats of water and iron are 4.184 j/goc and 0.44 j/goc respectively.
Problem \(\Pageindex{8}\) When 1.0 G Of Fructose, C 6 H 12 O 6 (S), A Sugar Commonly Found In Fruits, Is Burned In Oxygen In.
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry questions and problems 1. If the combustion of 0.285 moles of this compound causes the.