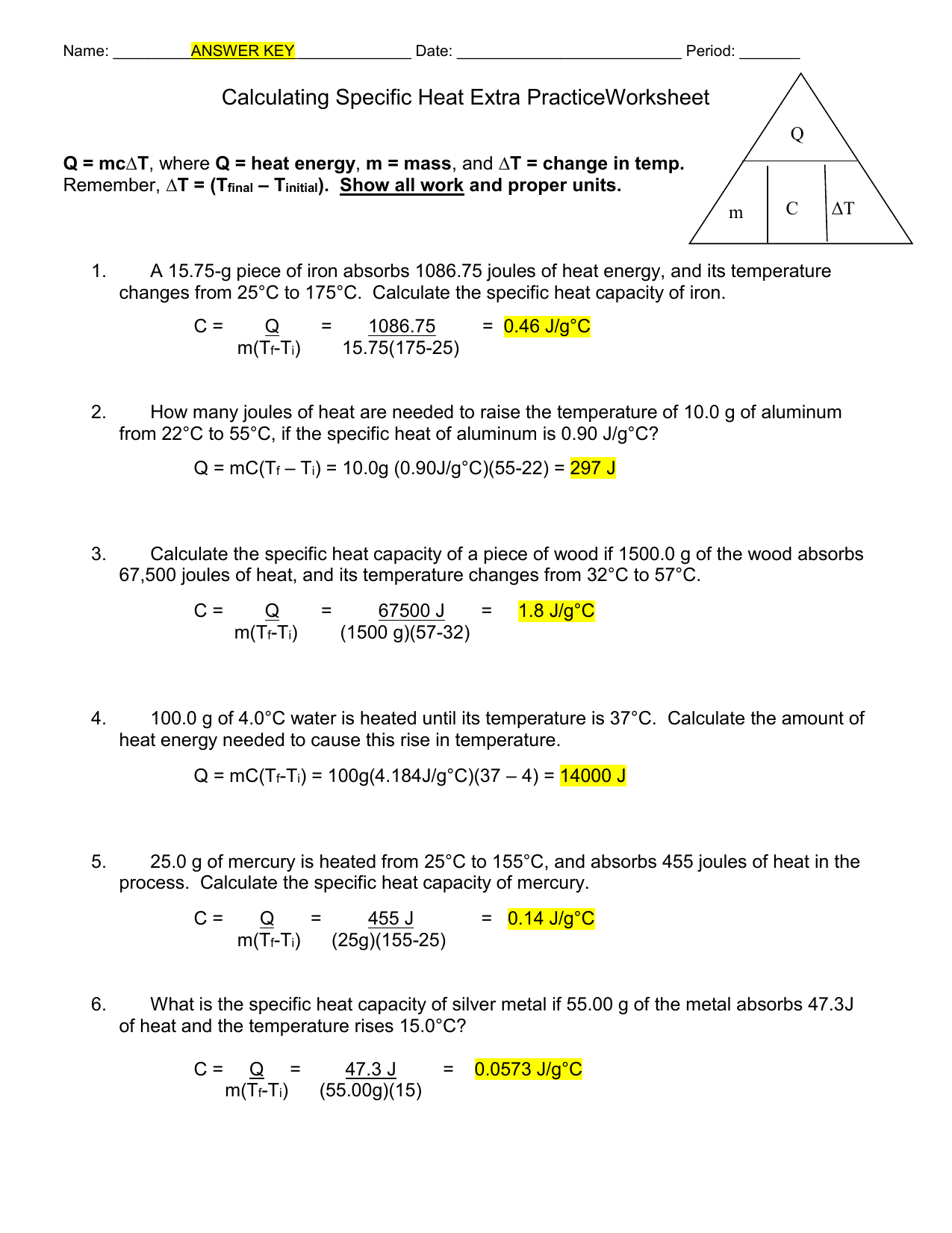
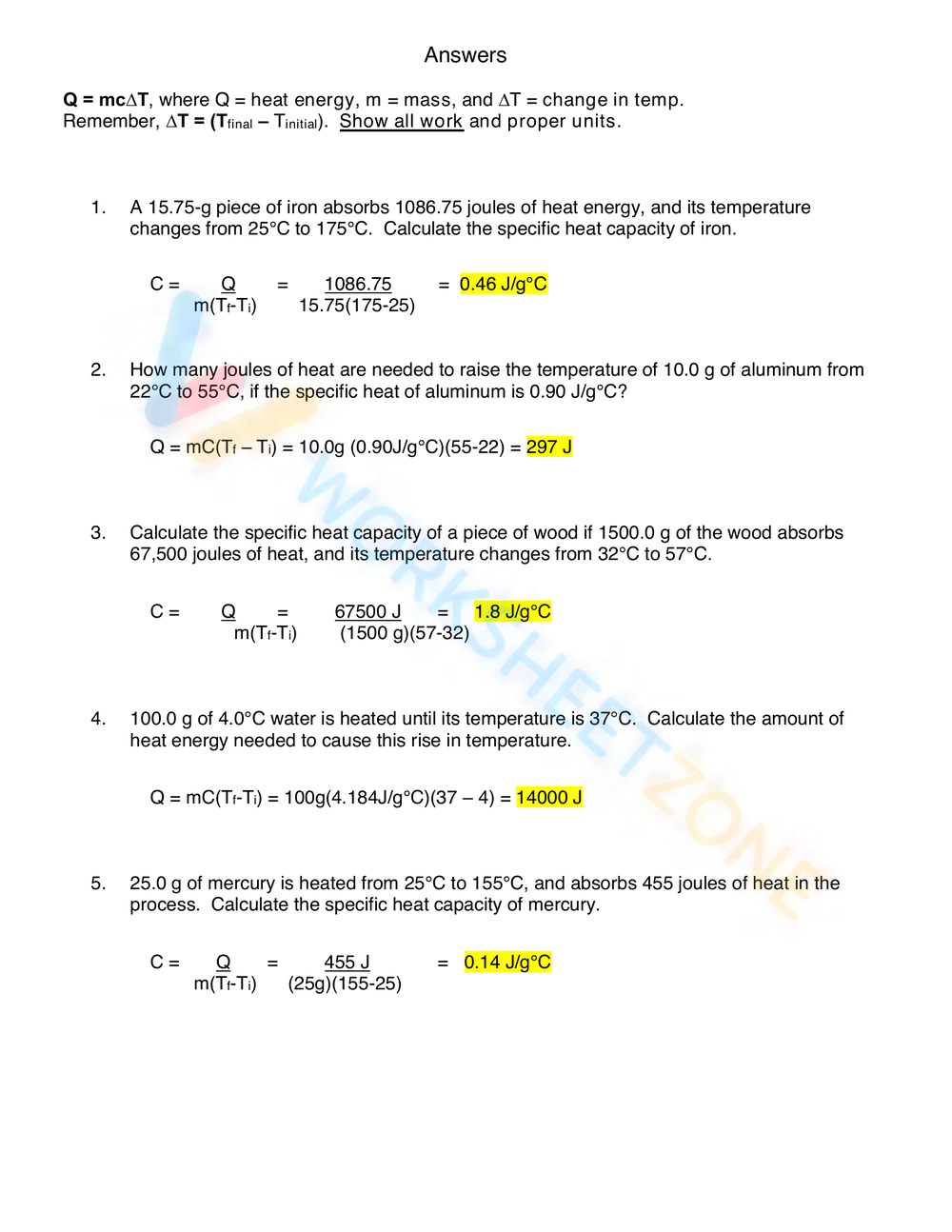
Calculating Specific Heat Extra Practice Worksheet - Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c.
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Use q = (m)(δt)(cp) to solve the following problems.
Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Show all work and units. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c.
Specific Heat Practice Worksheet at vangiavannablog Blog
Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculating heat specific heat is defined as the amount of.
Calculating Specific Heat Extra Practice Worksheet
Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating specific heat extra practice worksheet q = mc∆t, where q.
Calculating Heat And Specific Heat Worksheets
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Use q = (m)(δt)(cp) to solve the following problems. Show.
Heat Capacity and Specific Heat Calculations Practice A 1 g
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and units. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Use q = (m)(δt)(cp) to solve the following problems. Calculate.
Calculating Specific Heat Extra Practice Worksheet
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t.
Calculating Specific Heat Extra Practice Worksheet Doc Template pdfFiller
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating.
Specific Heat Practice Worksheet Printable Word Searches
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Show all work and units. Use q = (m)(δt)(cp) to.
Calculating Heat And Specific Heat Worksheet
Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature.
Extra practice calculating specific heat worksheet answers Studocu
Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of.
Calculating Heat And Specific Heat Worksheets
Show all work and units. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Use q = (m)(δt)(cp) to solve.
Show All Work And Units.
Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems.