Boyle S And Charles Law Worksheet - We are going to study 2 of the famous gas laws: What is the equation for boyle’s law?_____ 2. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. Boyle’s law worksheet fill in the blanks. What is the new volume? What does the letter p stand for? A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l.
What is the equation for boyle’s law?_____ 2. What is the new volume? We are going to study 2 of the famous gas laws: Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Boyle’s law worksheet fill in the blanks. What does the letter p stand for? A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l.
Boyle’s law worksheet fill in the blanks. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What is the new volume? Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. What does the letter p stand for? We are going to study 2 of the famous gas laws: A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. What is the equation for boyle’s law?_____ 2.
Gas Laws Presentation Chemistry
Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What does the letter p stand for? What is the equation for boyle’s law?_____ 2.
The Theories and Behavior of Gas Owlcation
What does the letter p stand for? We are going to study 2 of the famous gas laws: Boyle’s law worksheet fill in the blanks. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ temperature is held constant, the pressure and volume of a gas are.
Charles And Boyles Law Worksheet
What is the new volume? Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What is the equation for boyle’s law?_____ 2. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume.
Boyle's Law Questions And Answers
What does the letter p stand for? A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. When ____________ temperature is.
Worksheet Boyle's Law And Charles Law
A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. We are going to study 2 of the famous gas laws: Boyle’s law worksheet fill in the blanks. What does the letter p stand for? When ____________ is held constant, the pressure and volume of a gas are ____________.
Gas Laws Worksheet 2 Boyles Charles and Combined Gases Pressure
What is the new volume? Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. We are going to study 2.
What Does Boyle's Law Describe
When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. What is the new volume? Boyle’s law worksheet fill in the blanks. What is the equation for boyle’s law?_____ 2. We are going to study 2 of the famous gas laws:
Charle’s vs Boyle’s Gas Laws anchor chart Teaching chemistry
Boyle’s law worksheet fill in the blanks. What is the new volume? When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. What does the letter p stand for? When ____________ is held constant, the pressure and volume of a gas are ____________ proportional.
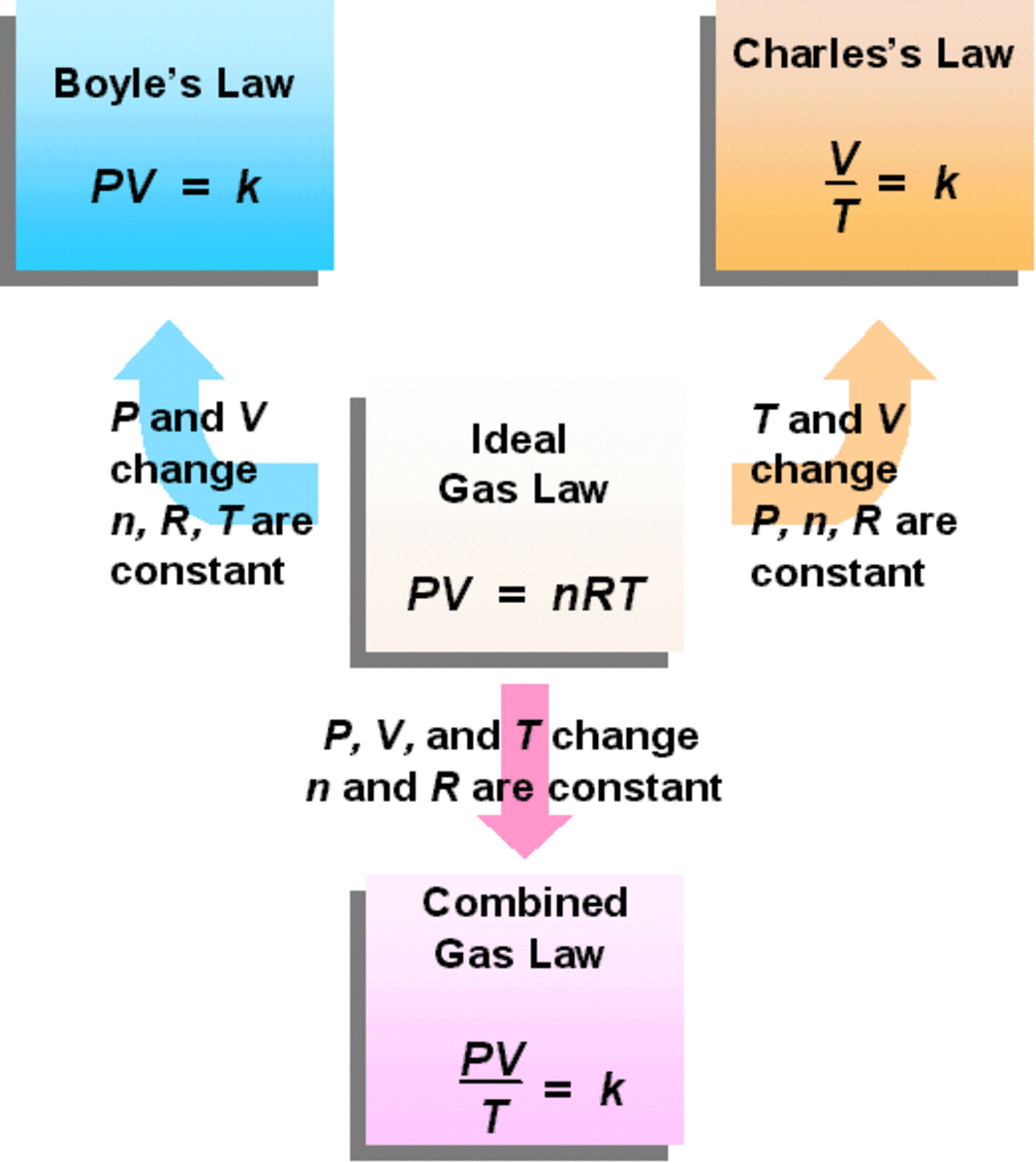
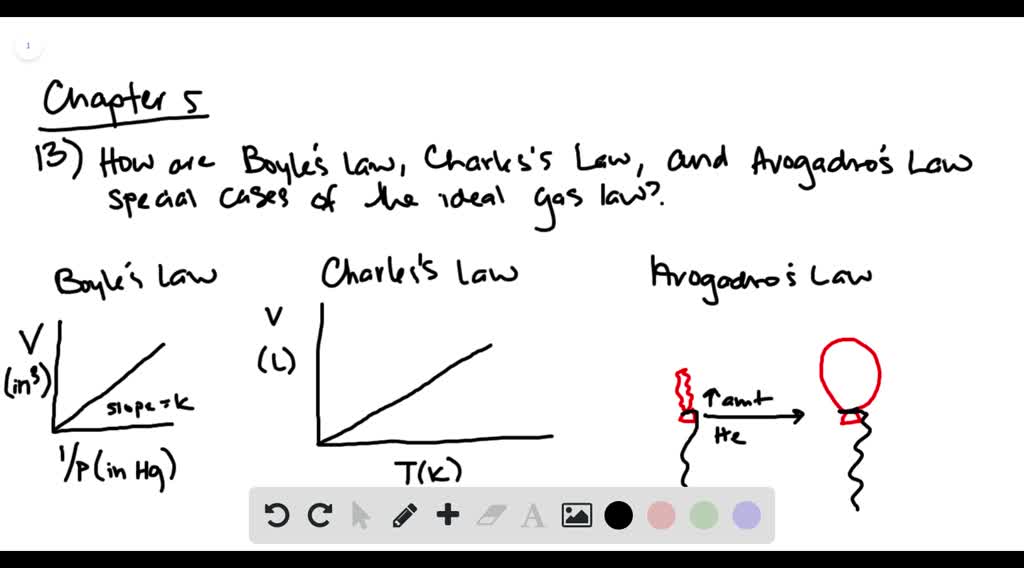
SOLVED Explain how Boyle’s law, Charles’s law, and Avogadro’s law are
We are going to study 2 of the famous gas laws: A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. What is the new volume? Boyle’s law worksheet fill in the blanks.
(DOC) Gas laws worksheet 2 boyles charles and combined (1)
What is the equation for boyle’s law?_____ 2. What does the letter p stand for? When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Boyle’s law, which looks at the relationship.
Boyle’s Law Worksheet Fill In The Blanks.
Boyle’s law, which looks at the relationship between pressure and volume, and charles’s. When ____________ temperature is held constant, the pressure and volume of a gas are ____________ inversely proportional. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the new volume?
What Does The Letter P Stand For?
What is the equation for boyle’s law?_____ 2. When ____________ is held constant, the pressure and volume of a gas are ____________ proportional. We are going to study 2 of the famous gas laws:
.PNG)